Scientists Heat Gold to 14 Times Its Melting Point: Imagine heating gold to a temperature nearly 14 times hotter than its usual melting point—over 19,000 kelvins (33,740°F)—and yet it stays solid. This jaw-dropping experiment recently conducted by scientists has flipped old physics theories on their heads and opened new doors to understanding matter under extreme conditions. Over decades, the melting point has been a key property of materials — like gold melting at about 1,337 kelvins (2,440°F). But heating something once it hits that point usually results in a liquid state. This new research shows if you heat gold fast enough, it defies that rule and stays solid far beyond what anyone believed possible.
Scientists Heat Gold to 14 Times Its Melting Point
Heating gold to 14 times its melting point without melting presents a stunning breakthrough in how we understand matter’s extreme limits. Ultrafast laser pulses keep gold solid by beating the clock on atomic movement, challenging decades of scientific dogma. This landmark experiment opens new paths for research in fusion power, planetary interiors, and materials science. It also pushes the frontier of ultrafast laser technology and precision measurements. If science has taught us anything, it’s that when things get extreme enough—and fast enough—nature often surprises us with new rules.

| Topic | Details |
|---|---|
| Temperature reached | 19,000 kelvins (33,740°F), 14 times gold’s melting point |
| Gold melting point | About 1,337 kelvins (2,440°F) |
| Heating method | Ultrafast laser pulses lasting 45 femtoseconds |
| Solid state maintained due to | Rapid heating preventing atomic rearrangement |
| Scientific importance | Challenges decades-old theory; helps understand matter at extreme temperatures |
| Applications | Fusion energy, planetary science, warm dense matter studies |
| Official Study Published in | Journal Nature, July 2025 |
| Official Website | SLAC National Accelerator Laboratory |
The Science of Melting and Entropy Catastrophe
Before diving into how this stunning experiment was done, let’s take a quick trip back in time. For decades, scientists believed materials couldn’t be superheated more than about 3 times their melting point without melting inevitably taking place. This was due to a mysterious phenomenon called entropy catastrophe.
Entropy is a measurement of disorder inside a system. For solids, atoms are arranged neatly in a crystal lattice, keeping entropy relatively low. As a solid heats and melts, atoms move around more freely, increasing entropy drastically. The entropy catastrophe theory says solids can’t stay orderly very long beyond certain temperatures because their entropy would surpass that of the liquid, which isn’t physically plausible.
Think of it like a school hallway. If students (atoms) stand quietly in line (solid state), things are ordered. But if they all start wandering around randomly (liquid state) beyond a point, it becomes chaotic (high entropy). The theory says the hallway can’t stay quiet if the heat makes everyone restless enough—yet gold broke this “rule” by staying orderly, literally too fast for chaos.
How Scientists Heat Gold to 14 Times Its Melting Point Without It Melting?
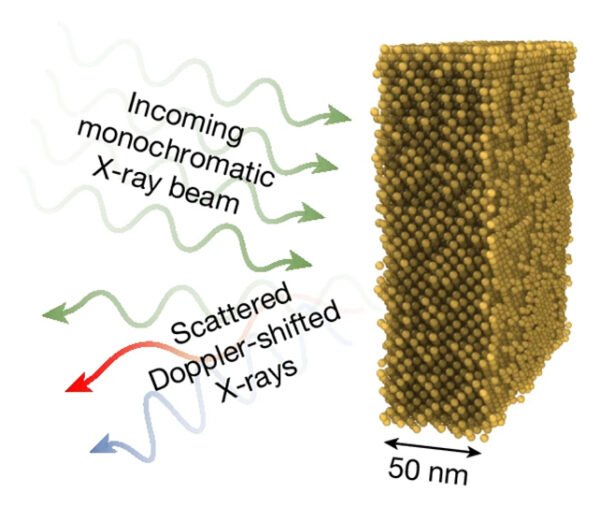
Researchers at SLAC National Accelerator Laboratory used extreme ultrafast laser pulses to heat super-thin layers of gold foil in mere femtoseconds (just 45 quadrillionths of a second!). This laser was an X-ray free electron laser that packs enormous energy into a split-second blast, rapidly heating then probing the atoms’ vibrations to measure temperature.
Since atoms didn’t have time to jostle into a liquid arrangement, the gold’s solid crystal structure remained intact, even at an impossibly high 19,000 kelvins. This powerful combination of rapid heating and precise measurement unlocked a new understanding of material physics.
The laser used, the Linac Coherent Light Source (LCLS) at SLAC in California, is almost two miles long and accelerates electrons to nearly the speed of light before producing one trillion X-ray photons in a pulse. This extreme power in a brief flash allowed scientists to heat the gold super quickly with stunning accuracy.
Why Scientists Heat Gold to 14 Times Its Melting Point Without It Melting Changes Everything?
Up until now, the entropy catastrophe theory limited how far scientists thought they could push superheating. This experiment debunks that long-standing view, showing that the rate of heating plays a crucial role—fast enough and you bypass meltdown.
This insight is crucial for science fields studying extreme states of matter such as in nuclear fusion—where plasma must be contained at incredibly high temperatures—and planetary science, where materials in cores of giant planets face massive heat and pressure.
Additionally, the superheated state lasted for about two picoseconds (two trillionths of a second), long enough for researchers to analyze and confirm this unexpected solid behavior. This suggests that under these ultra-fast conditions, solids might not even have a true melting point.
Real-World Applications
- Fusion Power: Helps design better containment and heating methods in fusion reactors to efficiently replicate sun-like energy on Earth.
- Planetary Science: Model the states of matter deep inside planets like Jupiter or exoplanets with extreme temperature and pressure environments.
- Materials Science: Guide future development of heat-resistant and exotic materials capable of surviving extreme environments.
- High-Energy Physics: Enhance understanding of warm dense matter that bridges the gap between solids and plasmas at high densities.
- Laser Technology: Demonstrates new capabilities of ultrafast lasers to probe matter in previously impossible ways, with potential uses in chemistry and condensed matter research.
The History of Melting and Superheating Research
Superheating isn’t completely new—scientists have known water can sometimes be heated beyond its boiling point without bubbling, like when microwaving water in very smooth containers. But solid superheating, especially to such extremes, was once thought impossible.
Back in the 1980s, physicists proposed that solids can only be superheated up to around three times their melting point—the so-called entropy catastrophe—beyond which solids would spontaneously melt because their entropy would exceed liquids, violating thermodynamic laws. For decades, no experiment seriously challenged this limit.
This new research shatters that assumption. The discovery shows the speed of heating can suppress atomic rearrangement, allowing solids to exist far beyond their predicted limits—potentially rewriting textbooks.
Challenges and The Road Ahead
While this experiment stunned the scientific community, challenges remain. The superheated state lasts only fleeting instants before the metal eventually cools or melts. Scientists still need to study how to maintain or utilize these conditions.
Future research plans to explore whether other metals and materials behave similarly under ultrafast heating and to fully understand how internal pressures or ionization might affect results.
Researchers are excited about testing different materials and pushing the limits of superheating further while also applying their new precise temperature measurement method to other high-energy-density physics problems.
Sheep Wool Might Just Be the End of Cavities; Scientists Stunned by Tooth Regeneration Discovery
It’s Not Gold or Platinum: The World’s Priciest Mineral Is Something You’ve Never Heard Of

















